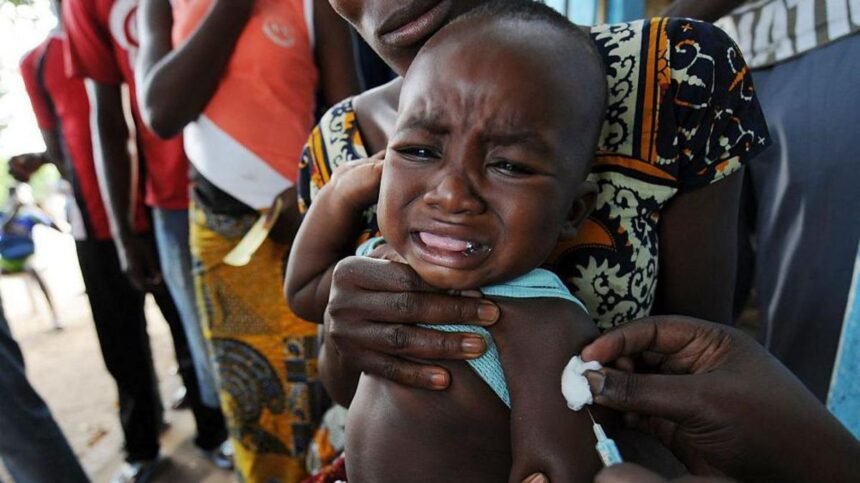
A proposed study funded by the United States aimed at testing the hepatitis B vaccine on newborns in Guinea-Bissau has been halted following strong criticism from the World Health Organization (WHO). The trial, which sought to administer the vaccine to some infants at birth while delaying it for others, was deemed unethical by the WHO, raising serious concerns regarding its scientific basis and ethical considerations.
The Controversial Trial
The $1.6 million initiative, financed by the US Centres for Disease Control and Prevention, aimed to involve 14,000 newborns in Guinea-Bissau. The plan was to administer the hepatitis B vaccine to one group at birth, while another group would receive the vaccine at six weeks of age. However, the WHO expressed “significant concerns” about the trial’s methodology, particularly its ethical safeguards and alignment with established research standards. The organisation emphasised that the hepatitis B birth dose is a vital public health intervention, proven to prevent transmission from mother to child in 70-95% of cases.
WHO officials highlighted that the hepatitis B vaccine has been in use for over three decades in more than 115 countries, stating that withholding such a life-saving intervention from newborns could lead to “potentially irreversible harm.” The organisation firmly advocates for all newborns to receive the vaccine within 24 hours of birth, as infection at this early stage is the primary pathway to chronic infection.
Public Outcry and Government Response
The trial faced immediate backlash from the local population, prompting the Guinea-Bissau government to suspend the project last month. Critics, including the country’s former health minister, Magda Robalo, voiced their disapproval, arguing that it is unacceptable to treat Guinea-Bissauans as experimental subjects. Robalo stated, “Guinea-Bissauans are not guinea pigs,” underscoring the ethical implications of conducting such a trial in a vulnerable population.

The controversy surrounding the trial intensified in light of recent developments in the US, where a panel of advisers—appointed by the US Health Secretary Robert F. Kennedy Jr.—recently ceased recommendations for universal hepatitis B vaccination for newborns. Critics have raised concerns about Kennedy’s previous statements questioning vaccine safety, further complicating the narrative surrounding the trial.
Implications for Hepatitis B in Guinea-Bissau
Hepatitis B is a significant public health issue in Guinea-Bissau, with estimates indicating that over 12% of the adult population is chronically infected. Some studies suggest that the prevalence could be as high as 20%. While many individuals may remain asymptomatic, chronic hepatitis B can lead to severe health issues, including cirrhosis and liver cancer.
Currently, the hepatitis B vaccine is administered at six weeks of age in Guinea-Bissau, but health authorities are working towards implementing the birth dose nationwide by 2028. The WHO has pledged to support this transition to meet global health standards.
Why it Matters
The cancellation of the hepatitis B vaccine trial in Guinea-Bissau serves as a crucial reminder of the ethical responsibilities inherent in medical research, particularly in low-income countries. It highlights the importance of ensuring that vulnerable populations are not exploited for experimental purposes, reinforcing the need for rigorous ethical standards in global health initiatives. As Guinea-Bissau navigates its public health challenges, the focus must remain on equitable access to proven interventions, safeguarding the health of its most vulnerable citizens.